Study Shows – Genes Aren’t Destiny of Inherited Blindness
February 4, 2026
Key takeaways:
- Inherited retinal degenerations (IRDs) were found by Mass General Brigham researchers to occur in only 28% of people who carry IRD genetic variants.
- Findings challenge conventional models of rare disease genetics, with implications for genetic testing and treatment.
A new study challenges what’s long been assumed about genetic variants thought to always cause inherited blindness. Investigators from Mass General Brigham used large public biobanks to determine that genes thought to cause inherited retinal degenerations (IRDs) with 100% certainty only led to disease in fewer than 30% of cases.
The findings, published in The American Journal of Human Genetics, challenge the traditional understanding of disease genetics for IRDs and other rare genetic diseases according to the researchers, and may have implications for the clinical use of genetic testing and the development of new treatments.
For more than a century, a central paradigm in genetics is that rare inherited disorders, often called Mendelian diseases due to discoveries by Gregor Mendel, are caused by misspellings in individual, critical genes. This paradigm has been supported by decades of studies in patients and families affected by many genetic disorders, including IRDs, which are the leading cause of legal blindness in working adults. Like other Mendelian diseases, IRDs were thought to be monogenic—with changes in a single gene always leading to the same physical disorder.
“Our study indicates that the number of people in the general population with genetic variants linked to inherited retinal disorders is much higher than previously thought, and population penetrance of these genes is markedly lower than traditionally assumed,” said senior and co-corresponding study author Eric Pierce, MD, PhD, director of Ocular Genomics Institute at Mass Eye and Ear, a member of the Mass General Brigham healthcare system, and Chatlos Professor of Ophthalmology at Harvard Medical School. “These findings are striking and suggest that the traditional paradigm of Mendelian diseases needs to be updated.”
Because most genetic studies typically take place in a clinic and include individuals and families already affected by disease, they may inflate estimates of disease penetrance. This hypothesis is known as ascertainment bias—or inadvertent selection for the most penetrant genetic variants and the most susceptible genetic backgrounds. The emergence of large volunteer biobanks that link genetic and clinical data offer new resources for genetic studies and enable more unbiased approaches to study rare disease genetics than previously utilized methods.
Mass Eye and Ear researchers used two large biobanks, the National Institutes of Health’s All of Us Research Program (AoU) and the UK Biobank (UKB), to determine how often certain genetic variants led to IRDs. They curated a list of 167 pathogenic variants in 33 genes that have previously been reported to cause IRDs. They then screened 317,964 AoU participants for these variants and identified 481 individuals with definite IRD-compatible genotypes.
Using strict International Classification of Diseases (ICD) diagnostic codes from electronic health record (EHR) data, the researchers found only 9.4% had an IRD diagnosis. Using a broader, more relaxed set of ICD codes that included other forms of retinal disease and vision loss, they found only 28.1% of individuals with the known genetic variants had the associated IRD.
To validate these findings, the team used data from the UKB, which captured retinal images for approximately 100,000 participants, providing researchers a different approach to determine evidence of retinal disease. Among individuals with IRD-associated variants, 16.1–27.9% showed definite or possible IRD features, closely aligning with estimates in the AoU dataset. Participant demographics, smoking, socioeconomic status, and comorbidities did not predict disease penetrance.
The findings indicate that additional genetic or environmental modifiers are required to manifest disease. This shift in understanding may impact how genetic testing is used in the clinic, and inform the development of novel therapies for IRDs and other genetic disorders. This new approach has been successful for disorders such as familial hypercholesterolemia.
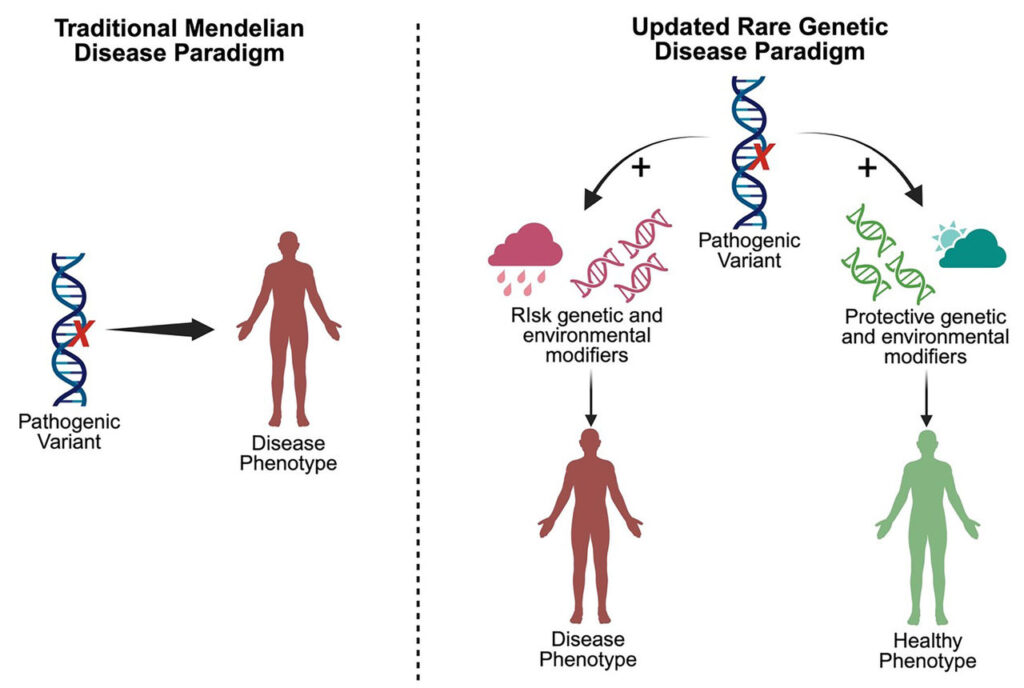
New findings from Mass General Brigham researchers indicate that rare genetic diseases like inherited retinal degenerations are not monogenic as they have been thought to be (left); rather, additional genetic or environmental modifiers are likely required to manifest disease (right). (Created in BioRender. Pierce, E. Mass Eye and Ear 2025)
“We think these findings are important for understanding IRDs and other inherited diseases,” said co-corresponding author Elizabeth Rossin, MD, PhD, an investigator at Mass Eye and Ear and assistant professor of Ophthalmology at Harvard Medical School. “We look forward to finding modifiers of disease and using that new knowledge to improve care for patients with IRDs and potentially other inherited eye disorders.”
Study limitations include recruitment biases to biobanks, and that the rare nature of IRDs limited the number of AoU and UKB participants with IRD-compatible genotypes. Future studies will seek to explore the penetrance of genetic variants associated with other Mendelian disorders, and begin the search for the other genetic factors which contribute to disease manifestation.
“The large number of individuals that do not develop an IRD despite having a compatible genotype provide an opportunity to design well-powered research studies to discover disease modifiers, which could spur development of novel therapies,” said study first author Kirill Zaslavsky, MD, PhD, who performed this research during his Inherited Retinal Disorders fellowship at Mass Eye and Ear.
Read the paper(opens external link in new tab)
Authorship: In addition to first author Zaslavsky and co-corresponding authors Pierce, and Rossin, additional Mass Eye and Ear co-authors include Liyin Chen, Chloe Park, Emily M. Place, Daniel Navarro-Gomez, Seyedeh M. Zekavat MD, PhD, Christopher F. Barile, and Kinga M. Bujakowska, PhD.
Disclosures: Disclosure forms provided by the author are available with the full text of the journal article at https://www.cell.com/ajhg/home.
Funding: This research was supported by an ACMG Foundation Next Generation Ophthalmic Genetics Fellowship Award (KZ), the Foundation Fighting Blindness Clinical Research Fellowship Award (KZ), a Research to Prevent Blindness career development award (EJR), grants from the National Eye Institute to EAP (RO1EY012910) and EJR (K23EY035342), and a P30 Core Grant for Vision Research (EY014104).
Paper cited: Zaslavsky, K et al. “Low population penetrance of variants associated with inherited retinal degenerations” Am J Hum Genet. doi.org/10.1016/j.ajhg.2025.11.015
Media contact
Ryan Jaslow
Program Director, External Communications (Research)
About Mass General Brigham
Mass General Brigham is an integrated academic health care system, uniting great minds to solve the hardest problems in medicine for our communities and the world. Mass General Brigham connects a full continuum of care across a system of academic medical centers, community and specialty hospitals, a health insurance plan, physician networks, community health centers, home care, and long-term care services. Mass General Brigham is a nonprofit organization committed to patient care, research, teaching, and service to the community. In addition, Mass General Brigham is one of the nation’s leading biomedical research organizations with several Harvard Medical School teaching hospitals. For more information, please visit massgeneralbrigham.org.
